Calculation of Activity at Constant Composition
Purpose: Learn to calculate activities of components as a function of temperature
Module: PanPhaseDiagram
Thermodynamic Database: AlMgZn.tdb
Batch file: Example_#1.11.pbfx
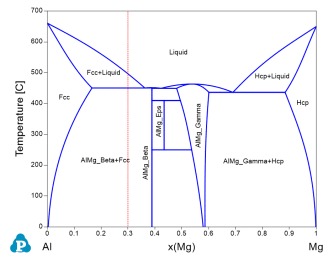
Figure 1 is the Al-Mg binary phase diagram. In this example, we learn to calculate the activity of Al and Mg as a function of temperature at a fixed composition (the red dash line).
Calculation Method 1, From menu bar “Property”:
-
Load AlMgZn.tdb following the procedure in Pandat User's Guide: Load Database , and select Al and Mg two components;
-
Click “Property” on the menu bar and select “Thermodynamic Property”;
-
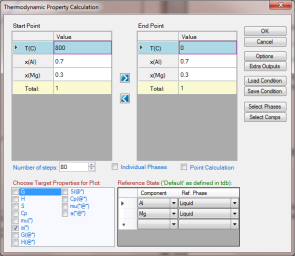
Set Calculation Condition as shown in Figure 2. The property selected is a(*), and the reference states are set as Liquid for both Al and Mg;
Calculation Method 2, From menu bar “PanPhaseDiagram”:
-
Load AlMgZn.tdb following the procedure in Pandat User's Guide: Load Database, and select Al and Mg two components;
-
Click “PanPhaseDiagram” on the menu bar and select “Line Calculation”;
-
Add the new table following the procedure in Pandat User's Guide: Icons for Table on Toolbar ;;
Post Calculation Operation:
-
Change graph appearance following the procedure in Pandat User's Guide: Property;
-
Add text and arrow on the plot following the procedure in Pandat User's Guide: Icons for Graph on Toolbar;
Information obtained from this calculation:
-
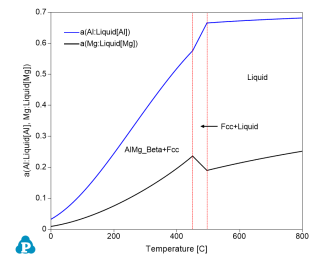
Figure 3 shows the activities of Al and Mg as a function of temperature using Liquid Al and Liquid Mg as reference states;
-
In the Liquid phase field at high temperature, activities of both Al and Mg decrease as temperature decreases;
-
In the Fcc+Liquid two-phase field, the activity of Al decreases as temperature decreases, but the activity of Mg increases as temperature decreases. This is because in this two-phase field the activity of each component follows its activity along the liquidus line. The composition of Mg increases along the liquidus line with decreasing temperature, which makes its activity increase;
-
In the AlMg_Beta+Fcc two-phase field, activities of both Al and Mg decrease with the decrease of temperature refer to the Liquid Al and Mg reference states;